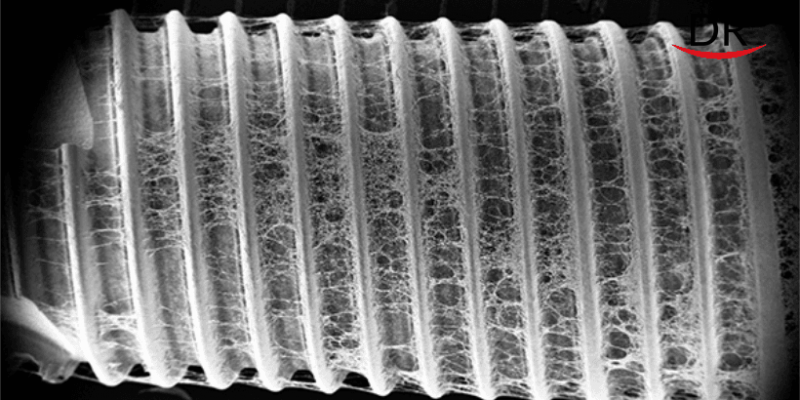
30 years after the first SEM research about contaminations on dental implants by Wahl et al., we still can find major impurities on many sterile implants today.
Not only clinical but also legal implications may occur in the future if practitioners are using implants of inferior quality. The quality of a dental implant is always the result of appropriate quality management.

Residues on sterile packaged implants, in particular organic particles from the production or packaging process, are highly suspected of being responsible for an incomplete osseointegration of dental implants or even a loss of bone in the early healing period.
While most of the failures of dental implants are related to osseointegration, implant making companies focus on patients with systemic clinical preconditions and even blame dentists for their lack of experience and training.
But there is another side of the coin too!
In three consecutive SEM studies, scientists of the University of Cologne and the Charité University Berlin have analysed more than 200 sterile packaged implants. Results from the most recent study and comparisons with previous years showed an alarming increase in implants with conspicuous residues.
Is your dental implant system actually sterile?
Neither the size and reputation of the manufacturer nor the country of manufacture can assure that implants are delivered as clean as promised or shown in advertising brochure
The CleanImplant Foundation has set the goal of providing precisely this information worldwide. This independent organisation is supported and managed by a scientific advisory board, which is chaired by renowned scientists and practitioners. In 2017, the board set the criteria for a new global quality seal, the CleanImplant Trusted Quality Mark.
Through a thorough protocol, implants are analysed in accredited testing laboratories according to DIN EN ISO/IEC 17025. This procedure guarantees objective results and reliable information for the new global quality seal.
Implant systems to date that meet the high standards of the Trusted Quality Mark are NobelActive (Nobel Biocare), V3 (MIS Implants Technologies), AnyRidge (MegaGen), UnicCa (BTI), SLA (Straumann), T6 (NucleOSS), blueSKY (bredent) and REPLICATE (NDI). Other implant systems are currently in the process of being analysed.
Practitioners interested in an appropriate answer to patients’ questions can join the CleanImplant Community and ask for a personalised certificate, which informs patients already in the practice waiting room. Implant manufacturers who want to apply for the quality mark can find more information on the project’s website, www.cleanimplant.com.
Beware of Lawsuits!
There have been many malpractice lawsuits filed by patients for whom dental implants were not successful.
For the patient, contaminants can induce an uncontrolled foreign-body reaction, resulting in periimplantitis, bone loss or even the failure of an implant and thus compromising the clinical outcome and the patient’s expectation.
Practitioners unknowingly using dirty implants have to deal with the risk of patient lawsuits for dental malpractice.


















Comments