– Dr Rashi Sharma
Introduction
As nations are easing off lockdowns, World Health Organization (WHO) has given various guidelines on lifting restrictions and emphasized on accelerating public testing. The number of cases of COVID-19 in India are more than 6,25,000 and the nation’s death toll is around 21,000 till date.
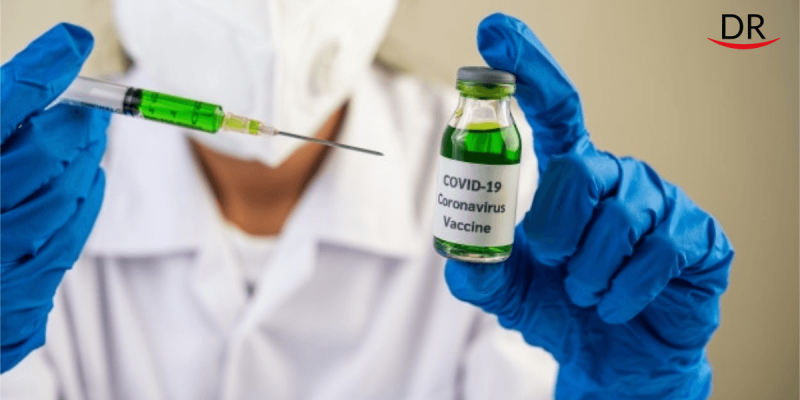
Indian Council of Medical Research (ICMR) has selected 12 institutes across the country for the clinical trial of India’s first COVID-19 vaccine. They have been asked to fast track clinical trials of the vaccine as it is the top priority of the nation, with an aim to launch the vaccine on August 15, India’s independence day.
Before knowing about the vaccine, we all should know about how a clinical trial of any new vaccine works.
What is a Clinical Trial?
Clinical trial is a systematic research in human subjects for evaluating the safety and efficacy of any new drug. Clinical vaccine trials include these phases:-
- Preclinical trials : These trials are usually done on animals to determine if the drug is safe enough for human testing.
- Phase I : In this phase, the drug is given to a small number of healthy and infected subjects to look for side effects and to figure out the best dose of administration.
- Phase II : In this phase, the drug is majorly given to hundred infected people to see whether it works or if there are any side effects that weren’t caught during the initial testing.
- Phase III : This is a large-scale trial, the drug is given to several hundred or even up to 3,000 people. A similar set of people take a placebo. The trial can take a few months to a few years. This stage provides the best evidence of how the vaccine works.
- Phase IV : In this phase, the vaccine that is approved for use, is continuously monitored to make sure there are no other side effects.
List Of Topmost Research Efforts Being Done Across The Globe Till Date Regarding COVID-19 Vaccine.
1. India’s double hope: Bharat biotech (COVAXIN) and Zydus Cadila (ZyCov-D)
The announcement of ZyCov-D by Zydus Cadila and COVAXIN by Bharat Biotech is the 'silver line in the dark clouds'.
Zydus Cadila has got a green signal from The Drug Controller General of India to start phase I and II of their clinical trials on human subjects. Zydus Cadila plants in Ahmedabad showed pleasing results in the pre-clinical trials which has paved the way for human trials.
COVAXIN, developed by Hyderabad-based vaccine manufacturer Bharat Biotech in collaboration with ICMR is all set to begin human clinical trials.
2. Plasma Therapy takes off in India
India got its exclusive ‘Plasma Bank’ in New Delhi recently. Delhi and Maharashtra are the topmost states who have approached ICMR for doing plasma therapy trials on patients. Researchers say treating infected patients with plasma from recovered people could reduce the number of coronavirus deaths. This therapy can be a breather for severely ill patients until a vaccine hits the market.
3. Glenmark Pharmaceuticals also launched an antiviral drug Favipiravir
Glenmark Pharmaceuticals also launched an antiviral drug Favipiravir under the brand name FabiFlu, for the treatment of patients with mild to moderate COVID-19 symptoms. Glenmark is also conducting another clinical trial to evaluate the efficacy of two antiviral drugs Favipiravir and Umifenovir as a combination therapy in infected patients in India.
4. Russia also begins human trials of COVID-19 vaccine
Russia has also started clinical trials of a vaccine, joining the global race to develop a safe COVID-19 vaccine. Officials said that the two forms of vaccine (liquid and powder) developed by the Moscow-based Gamaleya Research Institute of Epidemiology and Microbiology, will be tested on two groups of subjects involving 38 people each.
5. Britain introduces coronavirus vaccine squad
Britain introduced a new coronavirus squad to support efforts to make a vaccine available to the public as quickly as possible. This squad will be comprised of AstraZeneca, a multinational biopharmaceutical company , and the Wellcome Trust, a research-charity (14 million pound investment pool).
6. Oxford University's vaccine test shows promising results on monkeys
Researchers involved with the ChAdOx1 nCoV-19 trials said the vaccine had shown positive signs in monkeys and showed no indications of adverse effects. A single vaccination induced a humoral and cellular immune response in monkeys. Professor of Vaccinology, Sarah Gilbert at the University of Oxford's Jenner Institute who is leading the research, has shown full faith in this vaccine.
A Phase I/II human clinical trial of this vaccine, on 2000 participants consisting of South- African adults with and without HIV-infection, has begun since June 2020 with a primary completion date of October 2020.
7. America is likely to get a coronavirus vaccine before 2021
Moderna initially launched its vaccine test in mid-March with 45 human subjects. This company informed that it has finished enrolling 300 younger human subjects in its second stage of testing. It has already started studying how older adults react to the vaccine. These initial trials will check for side effects and how well people’s immune systems respond to different doses of the drug. But only huge trial can show if the vaccine works.
Colossal companies like Johnson & Johnson, Merck & Co., Pfizer Inc. and Moderna Inc. are taking part in the ‘Warp Speed’ program initiated by the Federal Government of the United States to facilitate and accelerate the development of COVID-19 vaccines.
Coronavirus Vaccine Becomes a Global Competition
India, Europe, America, Russia and China are battling to be the first to find a cure, bringing a nationalist element to a worldwide crisis. What began as a question of who would get the scientific accolades, the patents and ultimately the revenues from a successful vaccine have suddenly taken center stage. And behind the scramble is a harsh reality: Any new vaccine that proves potent against the coronavirus is sure to be in short supply as governments try to ensure that their own people are the first in line to get vaccinated.















Well done Dr Rashi, great information
Thanks alot
very well written Dr.Rashi..
informative Article..????????
Thanks alot ????
very well written Dr.Rashi
Thanks alot ????
Very informative…and gives some hope. Thanks for sharing Dr.Rashi.
Thank you so much for your kind words ????