Oral implants have a significant role in the rehabilitation of patients. Bone reconstruction techniques are essential to optimize the esthetic and functional outcome, and are often indicated to allow implant placement in an optimal 3D position to obtain long-term function and predictable esthetic outcome for prosthodontic restorations. The extent of ridge atrophy dictates whether the bone grafting is done with the implant placement or as a separate procedure.

Graft is basically a material, especially living tissue or an organ surgically attached to or inserted into a body part to replace a damaged part or compensate for a defect. In dentistry, evidence shows that grafting has been widely used for the management of
- craniofacial defects
- oral cancer
- congenital malformations
- infectious diseases
- cranioplasty
- surgical excision
- periodontal diseases
- trauma
- dental implantology
All these conditions can attribute to the development of big or small craniofacial deformities that require bone grafting. Recent advances in biotechnology have provided implant surgeons with access to many bone grafting materials, making implant treatment easier.
Rationale for bone grafts in implantology
Placement of implants requires sufficient bone volume and biologic quality. This is due to the macro design of the implant, which demands certain dimensional properties for long-term success. Other factors which make bone grafting necessary are:
- Resorption of the edentulous ridge post tooth extraction
- Presence of bony defects due to trauma or infection
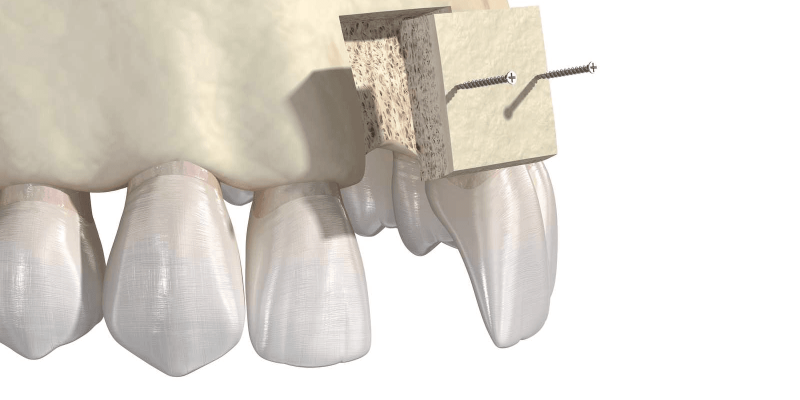
- The need to place implants in strategic sites for functional and esthetic success. In esthetic areas, soft tissue requires a bony base since “soft tissue follows hard tissue”.
Locations/Indications for bone grafts in implant treatment
Bone graft materials are placed in different locations for various indications:
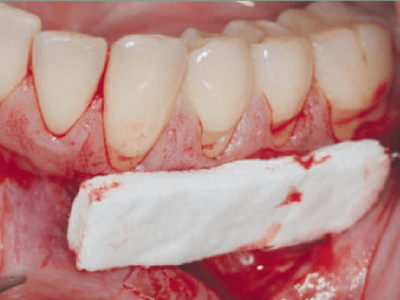
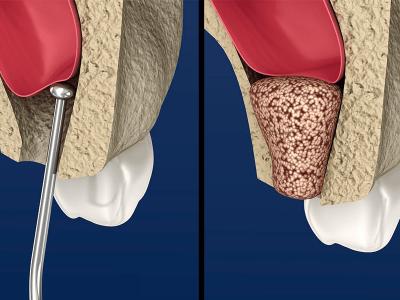
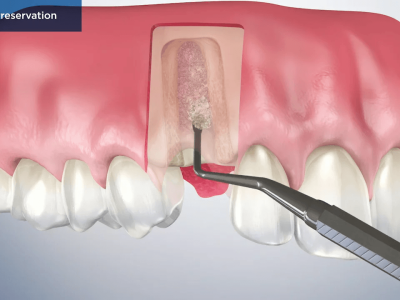
- In alveolar sockets post tooth extraction
- To fill a local bony defect due to trauma or infection
- To fill a peri-implant defect due to peri-implantitis
- For horizontal and vertical augmentation of the mandible and maxilla.
Read the requirements for the bone graft here!
Autograft
Autologous or autogenous bone grafting involves utilizing bone obtained from same individual receiving the graft. Types of autograft include osseous coagulum, bone blend, cancellous bone marrow transplant and bone swaging. Sources of bone include:
- iliac crest,
- mandibular symphysis,
- anterior mandibular ramus (coronoid process) and
- bone removed during osteoplasty and ostectomy.
Among the different available augmentation materials, only autologous bone combines osteoconductive, osteoinductive, and osteogenic characteristics compared to bone substitute and composite materials.
Autologous bone grafts have been considered as the “gold standard” and most effective material in bone regeneration procedures because of its properties and absence of immunological reactions. That is why, for block graft, autogenous bone is the most preferred because there is less risk of graft rejection as the graft is originated from the patient’s body.
Success rates exceeding 95% have been achieved, even when major augmentation procedures are carried out for severely resorbed jaws. However, limitations of intraoral autografts include:
- restricted donor sites
- possible harvesting morbidity
- reports of unpredictable resorption and
- limited available bone volume.
Allograft
The primary alternative to an autograft is the use of allograft materials, which can be obtained from either a compatible living donor or from cadaveric bone sources. Allograft materials can be prepared in three primary forms—fresh, frozen, or freeze-dried. Fresh and frozen allograft materials possess superior osteoinductive properties, but are rarely used nowadays due to the higher risk of a host immunogenic response, limited shelf life, and increased risk of disease transmission. Freeze dried bone allograft (FDBA) can be demineralized to reduce the above risks even further, giving rise to demineralized freeze dried bone allograft (DFDBA).
The advantages of allografts include
- availability in adequate quantities,
- sizes and shapes,
- predictable results and
- elimination of an additional donor site surgery.
Higher absorption rate, immunogenic response and less revascularization compared to autologous grafts has been reported among the disadvantages of this grafting category. Finally, due to the fact that a bone allograft is not a standardised tissue since age, gender and medical status of donors may vary in combination with existing diversity of processing procedures in bone banks, their properties may differ widely.
Xenografts
These materials derive from donors of a different species relative to the recipient, usually possess osteoconductive features with limited resorptive. Two illustrations of xenografts used in dentistry are:
1.Coral substitutes: Coral bone grafts have a geometry similar to that of human cancellous bone interconnected macropores (200-600 μm). They have been applied in jaw defects, exhibiting osteoconductive properties and functioning as carriers for growth factors, improving bone formation. They present initial poor mechanical strength, favorable to blood supply of recipient cite and fast resorption rate. Several studies have implemented them with encouraging results.
2.Bovine substitutes: Demineralized bovine bone grafts are biocompatible and osteoconductive. Bovine origin bone substitutes were the first xenografts applied to patients. They are commercially available in a wide range of products and considered among the most documented materials of this category. They have osteoconductive properties, being deproteinized and lyophilized, causing no immune response.
Synthetic bone substitute materials used in implantology include:
- Hydroxyapatite (HA)
- Tri-calcium phosphate (TCP)
- Calcium phosphate cement (CPC)
- Calcium sulfate
- Bone morphogenic protein (BMP)
Let us look at each one in detail.
Hydroxyapatite (HA)
HA is bioactive ceramic and a main mineral of bone. HA with a porous structure is easily bio-absorbable and exhibits good osteoconductivity. Therefore, when it is introduced in vivo, surrounding bone tissues grows and gradually progresses through the bone substitution. It can be inserted in line with a shape of a defective region. It is easily absorbed, does not generate metabolite impeding osteogenesis, and causes almost no foreign body reaction due to its excellent biocompatibility.
HA has very high compression and tensile strength compared with tri-calcium phosphate. Since HA is slowly degraded and retained in vivo for a long period of time, it impedes bone remodeling extends the mechanical vulnerability of new bone, and remains as permanent stressor.
Tri-calcium phosphate (TCP)
Tri-calcium phosphate is osteoconductive calcium phosphate and has the most similar chemical composition to human bone. It has better absorption than hydroxyapatite (HA). It is more porous than HA and features weak mechanical strength and fast absorption. More porous TCP undergoes biodegradation within 6 weeks after its introduction into the bone defect. Since its compression and tensile strength is very similar to that of cancellous bone, it is used in regions with no mechanical load.
As another main component, polyphosphate is highly concentrated in osteoblasts and is involved in mineralization of bone metabolism. In contrast of HA, ceramic TCP is biodegraded fast in vivo. It is biodegraded within 4–8 weeks after graft, and it is difficult to obtain proper bone formation during the early period. In consideration of these properties, biphasic ceramic with a mixture of HA and TCP is manufactured. Depending on the mixture ratio of these two components, it is possible to adjust the speed and degree of absorption and mechanical strength.
Calcium phosphate cement (CPC)
CPC consists of calcium phosphate. Calcium phosphate cements (CPCs) are frequently used to repair bone defects. Currently, CPC are defined as a combination of one or more calcium phosphate powders which, upon mixing with a liquid phase, form a paste able to self-set and harden in situ in the bone defect site to form a scaffold. CPC has osteoconductivity; it is gradually absorbed in the bone remodeling process and is replaced by a new bone.
Calcium sulfate
Calcium sulfate is clinically used to fill defects, such as bone cavities, and segmental bone defect, and moreover expansion use for spinal fusion and even for filling of harvest site of autogenous bone. Through recrystallization, it becomes a solid material and gives mechanical stability to its inserted region. Calcium sulfate normally undergoes biodegradation within 6–8 weeks after its insertion into the bone defect.
Given its lack of porosity, calcium sulfate has limited osteoconductivity. Given its mechanical disadvantage and rapid resorption compared with calcium phosphate, calcium sulfate is not often used.
Bone morphogenic protein (BMP)
Urist et al. reported that the growth factors extracted from bone organic component were able to induce osteogenesis and named them bone morphogenetic proteins (BMPs). Many types of local growth factors are related to bone healing. Depending on similarities in composition, these growth factors are classified into approximately 20 multiprotein growth factor families or superfamilies. Among them, only BMPs that belong to the transforming growth factor superfamily are known to run all processes of new osteogenesis. Depending on their levels, BMPs have a steep dose-response curve. If a large amount of BMPs are injected, osteoinduction occurs early, and a considerable amount of bone is generated.
Read about harvesting and healing of bone grafts in implantology here!
Summary
The subject of bone grafts for implant procedures is complex and many a times confusing for the dental surgeon. Of the graft materials autogenous bone is the gold standard. However, there have been many shortcomings of autogenous bone material. To supplement autogenous bone, allogeneic bone is often used. However, depending on use conditions, the bone union rate of allogeneic bone is not satisfactory, and there is a potential for infectious diseases. For this reason, the development of synthetic materials has drawn a lot of attention and has been researched. Although synthetic grafts have advantages of easy production and no risk of infectious diseases, it has only osteoconductivity, causes mechanical vulnerability depending on material characteristics such as bioabsorption, and has difficulty playing its role continuously in the process of bone union.
Currently, there is no material to completely replace autogenous bone, and it is not easy to select the best bone substitute. Therefore, in selecting the material, tissue survival capability, the size of bone defect, the size and shape of the graft material, biomechamical properties, ease of manipulation, cost, ethical issues, biological properties, and complications must be considered; biological and mechanical characteristics must be evaluated in each clinical situation. Though nowadays recent advances of zygomatic and pterygoid implants are also substituting the use of grafts vey less, though it requires a more clinical skill and is slightly controversial.
References
1. Jensen AT, Jensen SS, Worsaae N. Complications related to bone augmentation procedures of localized defects in the alveolar ridge. A retrospective clinical study. Oral Maxillofac Surg. 2016;20(2):115–122. doi: 10.1007/s10006-016-0551-8. [PubMed] [CrossRef] [Google Scholar]
2. Buser D, Dula K, Hirt HP, Schenk RK. Lateral ridge augmentation using autografts and barrier membranes: clinical study with 40 partially edentulouspatients. J Oral Maxillofac Surg. 1996;54:420–432. doi: 10.1016/S0278-2391(96)90113-5. [PubMed] [CrossRef] [Google Scholar]
3. Elsalanty ME, Genecov DG. Bone grafts in craniofacial surgery. Craniomaxillofacial trauma reconstruction. 2009;2(3):125-34.
4. Kumar P, Vinitha B, Fathima G. Bone grafts in dentistry. J pharmacy bioallied sci. 2013;5(1):S125- 7.3. Galindo-Moreno P, Avila G, Fernández-Barbero JE, Mesa F, O’Valle-Ravassa F, Wang HL. Clinical and histologic comparison of two different composite grafts for sinus augmentation: a pilot clinical trial. Clin Oral Implants Res. 2008;19:755–759. doi: 10.1111/j.1600-0501.2008.01536.x. [PubMed] [CrossRef] [Google Scholar]
5.Galindo-Moreno P, Avila G, Fernández-Barbero JE, Mesa F, O’Valle-Ravassa F, Wang HL. Clinical and histologic comparison of two different composite grafts for sinus augmentation: a pilot clinical trial. Clin Oral Implants Res. 2008;19:755–759. doi: 10.1111/j.1600-0501.2008.01536.x. [PubMed] [CrossRef] [Google Scholar]
6. Chiapasco M, Zaniboni M, Rimondini L. Autogenous onlay bone grafts vs. alveolar distraction osteogenesis for the correction of vertically deficient edentulous ridges: a 2–4-year prospective study on humans. Clin Oral Implants Res. 2007;18:432–440. doi: 10.1111/j.1600-0501.2007.01351.x. [PubMed] [CrossRef] [Google Scholar]
7. Moses O, Nemcovsky CE, Langer Y, Tal H. Severely resorbed mandible treated with iliac crest autogenous bone graft and dental implants: 17-year follow-up. Int J Oral Maxillofac Implants. 2007;22:1017–1021. [PubMed] [Google Scholar]
8. Zizelmann C, Schoen R, Metzger MC, Schmelzeisen R, Schramm A, Dott B, Bormann KH, Gellrich N-C. Bone formation after sinus augmentation with engineered bone. Clin Oral Implants Res. 2007;18(1):69–73. doi: 10.1111/j.1600-0501.2006.01295.x. [PubMed] [CrossRef] [Google Scholar]
9. Sbordone C, Toti P, Guidetti F, Califano L, Pannone G, Sbordone L. Volumetric changes after sinus augmentation using blocks of autogenous iliac bone or freeze-dried allogeneic bone. A non-randomized study. J Craniomaxillofac Surg. 2014;42:113–118. doi: 10.1016/j.jcms.2013.03.004. [PubMed] [CrossRef] [Google Scholar]
10. Stricker A, Schramm A, Marukawa E, Lauer G, Schmelzeisen R. Distraction osteogenesis and tissue engineering-new options for enhancing the implant site. Int J Periodontics Restorative Dent. 2003;23(3):297–302. [PubMed] [Google Scholar]
11. Nkenke E, Neukam FW. Autogenous bone harvesting and grafting in advanced jaw resorption: morbidity, resorption and implant survival. Eur J Oral Implantol. 2014;7:203–217. [PubMed] [Google Scholar]
12. Klein MO, Al-Nawas B. For which clinical indications in dental implantology is the use of bone substitute materials scientifically substantiated? Eur J Oral Implantol 2011;4:11- 29.
13. Schug J, Kirste M, Huber A, Hollay HC, Troedhan A, Leventis MD et al. Post extraction alveolar ridge preservation: scientific background, minimally invasive treatment protocols and expert reports using alloplastic biomaterials. Sunstar Guidor 2014;1.
14. Laurencin C, Khan Y, El‑Amin SF. Bone graft substitutes.Expert Rev Med Devices 2006;3:49‑57.
15. Mulconrey DS, Birdwell KH, Flynn J, Cronen GA, Rose PS. Bone morphogenic protein (RhBMP‑2) as a substitute for iliac crest bone graft in multilevel adult spinal deformity surgery: Minimum two‑year evaluation of fusion. Spine (Phila Pa 1976) 2008;33:2153‑9.
16. Kumar P, Vinitha B, Fathima G. Bone grafts in dentistry. J Pharm Bioallied Sci 2013;5(1):125-7.
17. Sakkas A, Wilde F, Heufelder M, Winter K, Schramm A. Autogenous bone grafts in oral implantology—is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int J Implant Dent 2017;23.
18.Titsinides S, Agrogiannis G, Karatzas T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. Japanese Dent Sci Rev 2018;55(1):26-32.
19. Hiatt WH, Schallhorn RG. Intraoral transplants of cancellous bone and marrow in periodontal lesions. J Periodontol 1973;44:194-208.
20. Robinson E. Osseous coagulum for bone induction. J Periodontol 1969;40:503-10.
21. Diem CR, Bowers GM, Moffitt WC. Bone blending: A technique for osseous implants. J Periodontol 1972;43:295-7
22. Shibuya N, Jupiter DC. Bone graft substitute: allograft and xenograft. Clin Podiatr Med Surg 2015;32:21-34.
23. Venkataraman N, Bansal S, Bansal P, Narayan S. Dynamics of bone graft healing around implants. J Int Clin Dent Res Organ 2015;7(3):40.
24. Hoon-Sang Sohn, Jong-Keon Oh. Review of Bone graft and bone substitutes with an emphasis on fracture surgeries.Biomaterials Research 2019; 23:9
Author: Dr Priti Jaiswal















Comments