This article is the winning entry of the DRDCA 2020 Article Contest (Third Position). Congratulations to the author Dr. Veena Pai!
INTRODUCTION
Adhesive system can be categorized on how they treat the smear layer. The traditional total-etch system removes smear layer and smear plugs and demineralise the subsurface dentin which is replaced by adhesive monomer. This is not always possible owing to the sensitivity of technique. Slightest variation in the steps involved – etching, rinsing, drying etc resulted in a hybrid layer that contained voids, usually seen at the bottom of hybrid layer. [1]
In order to simplify and overcome the sensitivity of the wet bonding technique, self-etch systems were introduced. Although the bond strengths are not high as two step systems as reported in many in-vitro studies, single step adhesives have an advantage of simple bonding procedure with reduced technique sensitivity. The greatest concerns regarding self-etch adhesive systems refers to whether they are acidic enough to produce adequate demineralization. The pH is a key factor to determine the penetration potential throughout dentin and depth of demineralization. [2]
It has been reported that although these self-etch adhesives are highly acidic, the degree of conversion (DOC) of this acidic monomer at the surface and within the tubules is high enough for the acidic reaction to be self limiting. However there are certain controversial reports to show that the acidity of the unpolymerized monomer may be retained and continue to affect or demineralise the surrounding dentin, resulting in unprotected collagen fibrils susceptible for hydrolytic degradation[3]. Therefore the self-etch concept although theoretically appears perfect, it needs to be thoroughly analysed for its acidity and effect on wet, fluid-filled dentinal tubules. The study was undertaken with the null hypothesis that there was no significant difference between single step and two step self etch adhesive (SEA) with respect to their 1) degree of conversion(DOC) and 2) maintenance of dentinal integrity with the objective to determine whether the unpolymerized acidic monomer in the fluid filled dentinal tubules, would retain their acidity and continue to etch or demineralize the surrounding dentin.
MATERIALS AND METHODS:
Methodology:
Dentin Adhesive (D/A) Specimen Preparation:
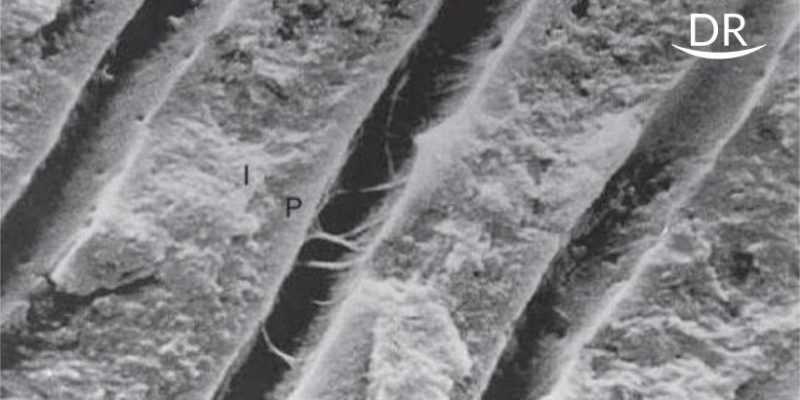
Occlusal one third of intact non-carious molars was removed by a diamond disk and a smear layer was created. The prepared dentin surfaces were divided into following groups and sectioned longitudinally, into four rectangular slabs (8 x 1.5 x 1.5 mm) having a dentin-adhesive inter face.
Group 1. Single bottle SEA- G Bond (10 Molars)
Group 2: Two bottle SEA- Unifil Bond ( 10 Molars)
Two slabs from one tooth were analysed after 24 hrs under magnetic resonance spectroscopy (MRS) for the DOC at the D/A surface, 10µm and 20µm into dentin. The chemical structure of bonded dentin after 24 hrs was analysed to compare with the four weeks specimen, to measure if any demineralization has occurred (Graph 1). Two slabs from same tooth were analysed after 4 weeks under MRS to determine whether the residual monomer, if any, would alter the chemical structure of dentin.
Quantifying the Spectral Readings:
Spectral data from the data interfaces were compared with reference spectra of pure adhesive and normal dentin.
Evaluation of Degree of conversion
Group 1 (G Bond), the DOC was measured in MRS by measuring intensity ratio (R) of the vibration bands of aliphatic C=C stretching mode at 1567 cm-1 and aromatic C=C stretching mode at 1422 cm-1used as internal standard. Readings were calculated at D/A surface, 10µm, and 20µm into dentin (Graph 1b,2a)
Group 2 (Unifil Bond), the DOC was measured in MRS by intensity ratio (R) of the vibration bands of aliphatic C=C stretching mode at 1410 cm-1 and the aromatic C=C stretching mode at 1245 cm-1 used as internal standard. Readings were calculated at D/A surface, 10µm, and 20µm into dentin.
Evaluation of demineralization and degradation of dentin
The intensity of peak 960 cm-1 representative of mineral content of normal dentin by PO4 vibration, and 1666cm-1 representative of organic content of dentin by amide I were taken as standards (Graph 1a) to compare readings of same peaks after bonding and after four weeks storage to analyse the changes in the organic and inorganic component in the dentin samples (Graph 2d, 3d).
RESULTS AND DISCUSSION
The DOC of adhesives is an important clinical parameter since low mechanical properties are related with low percentage of monomer to polymer conversion. On comparing the DOC between two groups, no significant difference was found between the groups (Graph 4). This result is in conformity with most of the studies comparing the degree of conversion of adhesives using one-step [3] and two-step adhesive.
The results also indicates that in the deeper layer of hybrid layer, there is increased probability of incomplete conversion irrespective of the number of steps involved. As discussed by Carvalho in his review on SEAs, the highly hydrated dentin substrate as it nears the pulpal chamber might interfere with the polymerization as well as penetration of the monomer into the tubules. [4] DOC has been shown to vary with the technique of application, curing method, curing unit used and also the composition of the adhesive. Simplified adhesives with 50% of solvents in their composition have shown to lower the degree of conversion.
The second part of study evaluated the integrity of adhesive treated dentin substrate after 4 weeks. Dentin substrate of 18% of G-Bond group (Graph 2d) and 11% of Unifil group specimens (Graph 2d), which were intact initially (24 hrs specimen analysis) showed mineral loss after 4 weeks. This was indicated by the decreased intensity of the phosphate (960 cm-1) and amide peak (1666cm-1), confirming demineralization and degradation at 20µm. The Z test done for proportionality within each group, showed statistically significant degradation in both the groups indicating continued demineralization over time (Table 1). However there was no statistically significant difference (p > 0.05) between the groups tested in this study thus accepting the null hypothesis (Table 2), which is in agreement with the study by Wang et al. [3]
Few studies suggest that in adhesives with pH < 1, the smear layer is dissolved and the tubules are opened up resulting in dilution and phase separation of adhesive solution, decreasing the tendency of hydrophilic and hydrophobic monomer to polymerize as copolymers. The unpolymerized monomer remains acidic and continues to etch the underlying dentin after hybrid layer formation. Kitasako et al concluded that the mild acidic self etch monomers are neutralized as they bind and dissolve calcium and phosphate ions from the tooth surface making the acidic action self-limiting thus causing no damage to the substrate which is in contrast to our results. The SEA used in the present study were of pH 2.0 (G Bond) and pH 2.2 (Unifil Bond), which although considered mild was less than that used by Kitasako et al.[5] Therefore the effect of different pH and its relation to the demineralization of dentin and polymerization of adhesives needs to be further evaluated.
While nanoleakage has been reported with both total-etch and self-etch adhesives, the latter seem to have shown greater degree of nanoleakage owing to the higher content and hydrophilicity of the solvents. This makes the hybrid layer more porous and more water is absorbed into the polymer overtime causing swelling of resin by hydrogen bonding of water on hydrophilic domains of polymer chains. The residual unreacted monomer is eluted from hybrid layer creating new channels of water penetration, through which the water diffuses even more in an auto-accelerative manner. The elution of residual monomer and low weight polymers also cause an increase in porosity within hybrid layer and lower the cohesive strength of adhesive. The resulting bond strength value is also lowered thus weakening the D/A bond. Moreover the D/A bond is rendered unstable over a period of time due to demineralization of dentin.
Tay et al showed that it is difficult to evaporate water from all-in-one adhesives, and even if evaporation is successful, water will rapidly diffuse back from the bonded dentin into the adhesive resin. This water sorption plasticize polymers and lowers their mechanical properties. Although hydrophobic dimethacrylate are added to all-in-one adhesives to produce stronger cross-linked polymer networks, the hydrophilic monomers tend to cluster together before polymerization to create hydrophilic domains and microscopic water-filled channels called “water trees.” These ‘water trees’ permit movement of water from the underlying dentin resulting in degradation of bond. [6]
Biodegradation of the collagen matrix and/or hydrophilic resin components within the hybrid layer may be related to: (1) Incomplete infiltration of resin into the dentine (2) Heterogeneous distribution of resin monomers through the interdiffusion zone (3) Suboptimal polymerization in the presence of water (4) Alterations of the organic matrix during preparatory procedures (5) Hydrolysis of polymeric components or unprotected collagen.
A study concluded direct exposure to water affected bonds produced by two step total-etch adhesives. [7] In the present study though the specimens were directly exposed to water, the storage period was only 4 weeks. In the oral cavity as dentin bonding agents are generally covered by restorative resins or other restorations, direct exposure can hardly be expected unless in cases of marginal microleakage. Hence further long term in-vivo and in-vitro studies addressing these aspects should be conducted.
Several reasons have been advocated to account for the suboptimal bonding performance of these simplified adhesives: (1) the more aggressive etching process, which may destabilize the collagen matrix (2) a weaker cohesive strength of the adhesive resins (3) a low degree of conversion of the resin monomer in these simplified adhesives (4) the formation of ‘‘water-trees’’.
CONCLUSION
The following conclusions could be drawn from the present study
- DOC of the monomer to polymer at the surface was significantly higher than that found deeper within the tubules in both single and two step adhesives.
- There was no significant difference in the DOC between the two groups tested (Graph 4).
- Both single step and two-step SEA showed statistically significant degree of continued demineralization and collagen degradation after 4 weeks of water storage (Table 1).
- There was no statistically significant difference in the demineralization and collagen degradation after 4 weeks of water storage between the groups tested (Table 2).
- Continued demineralization and degradation could be attributed to the unpolymerized acidic monomer within the hybrid layer.
- The relationship between the variables like pH, technique of application, DOC, storage of specimen & adhesive, composition of adhesives and its’ effect on the degradation of the D/A interface should be further evaluated through long term in-vitro and in-vivo studies.
FIGURES & TABLES
Results after MRS analysis
Spectral Readings Used As Standards-Graph 1

Graph 1a – MRS spectra obtained for normal dentin -peaks at 960cm-1(phosphate) and 1666cm-1 (amide I) used as internal standards.

Graph 1b- MRS spectra obtained from uncured G Bond adhesive system. Intensity of peaks- 1657 cm-1 and 1422 cm-1 used as internal standards.

Graph 1c- MRS spectra obtained from uncured Unifil Bonding agent. Intensity of peaks- 1410 cm-1 and 1245 cm-1 used as internal standards.
Raman spectra of G bond group ( graph 2)

Graph 2a – Spectral readings of cured samples at three different depths after 24 hrs.

Graph 2b -Specimens showing no penetration at 20 micro-m.

Graph 2c – showing peak intensities similar to the uncured resin at 20micro-m indicating sub-optimal polymerization.

Graph 2d – specimens which showed suboptimal polymerization, show reduced intensity of peak 960cm-1, indicating demineralization at 20micro-m.

Raman spectra of Unifil bond group ( graph 3)

Graph 3a – Spectral readings of cured samples at three different depths after 24hrs.

Graph 3b – group 2 specimens showing no penetration. Note the intensity of peak 1245cm-1 and 1410cm-1 at 20micro-m.

Graph 3c – Unifil bond group showing peak intensities similar to the uncured resin indicating suboptimal polymerization at 20micro-m.

Graph 3d – specimens which showed suboptimal polymerization, showing demineralization indicated by the reduced intensity of peak 960cm-1 at 20 micro-m.
Statistical Analysis – Bar graph showing Degree of Conversion in Group 1 and Group 2
(Graph 4)

REFERENCES
1) Cem sahin, Zafer C. Cehreli, Muhittin Yenigul and Bulent Dayangac. In vitro permeability of etch-and-rinse and self-etch adhesives used for immediate dentin sealing. Dental Materials Journal 2012; 31(3): 401–408.
2) R.Vinaychandra. Self-etch Adhesives: Simple, Easier… but is it Better?. JIOH, August 2010, Volume 2 (Issue 2);85-91.
3) Wang V, Spencer P. Continuing etching of our all in one adhesive in wet dentin tubule. Journal of Dental Research 2005; 84(4): 350-355.
4)Carvalho RM, Chersoni S, Frankenberger R, Pashley DH, Prati C,Tay FR. A challenge to the conventional wisdom that simultaneous etching and resin infiltration always occurs in self-etch adhesives. Biomaterials 2005; 26: 1035-42.
5)Kitasako Y,Burrow M F,Nikaido T,Tagami J. The influence of storage on dentin bond durability of resins. Dental Material 2006; 16: 1-6.
6) Tay FR, Pashley DH. Aggressiveness of contemporary self etching systems. I. Depth of penetration beyond dentin smear layers. Dental Materials 2001; 17: 296–308.
7) J. De Munck, B. Van Meerbeek, Y. Yoshida, S. Inoue, M. Vargas, K. Suzuki, P. Lambrechts, and G. Vanherle. Four-year Water Degradation of Total-etch Adhesives Bonded to Dentin. Journal of Dental Research 2003; 82(2):136-140.
7) Franklin R. Tay, David H. Pashley. Have Dentin Adhesives Become Too Hydrophilic?. Journal of Canadian Dental Association 2003; 69(11): 726–31.


















Comments