KYOTO: A groundbreaking drug capable of regrowing teeth is set to begin human trials in September 2024, marking a significant advancement in dental medicine. Developed by researchers at Kitano Hospital in Japan, this innovative treatment aims to regenerate teeth by targeting a protein that inhibits tooth growth.
Development and Mechanism: The drug, spearheaded by molecular biologist and dentist Katsu Takahashi, deactivates the uterine sensitization-associated gene-1 (USAG-1) protein, which suppresses tooth growth. By blocking USAG-1, the drug promotes bone morphogenetic protein (BMP) signaling, triggering the generation of new bone and teeth. Takahashi has dedicated his research to tooth regeneration since 2005, aiming to assist those suffering from tooth loss or congenital deficiencies. The drug has shown success in animal models, including mice and ferrets, without significant side effects.
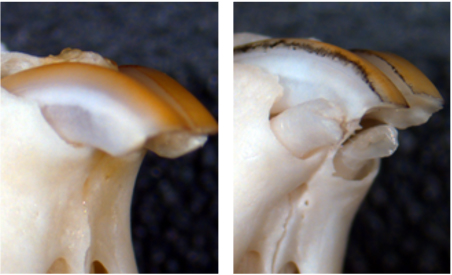
In mice deficient in USAG-1, an antagonist of BMP, the trace deciduous incisors survive and erupt as excess teeth (Kyoto University/Katsu Takahashi)
Clinical Trial Phases: The clinical trials will be conducted at Kyoto University Hospital, beginning with 30 males aged 30-64 who are missing at least one molar. This phase will run from September 2024 to August 2025. Subsequent trials will focus on children aged 2-7 with congenital tooth deficiency, a condition affecting about 1% of the population. A third phase will target older adults missing one to five permanent teeth due to environmental factors.
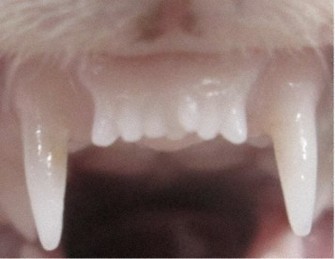
This photo provided by Kitano Hospital shows the front teeth of a ferret that was given the medicine. It had six teeth at first, but a seventh one, pictured at center, grew in.
Target Demographics: The drug has the potential to benefit various groups, including:
- Individuals with congenital tooth deficiencies, such as oligodontia, affecting 0.1% of the population.
- People who have lost teeth due to cavities, injuries, or other environmental factors. It is estimated that around 5% of Americans are missing teeth, with a higher prevalence among older adults.
Commercial Availability Timeline: If clinical trials are successful, the drug could be available by 2030, according to researchers at Toregem Biopharma, a Kyoto University-affiliated startup. Initially, the treatment will be offered to patients with congenital anodontia, before expanding to those who have lost teeth later in life. This development could revolutionize dental care by providing a permanent solution to tooth loss.
Lead researcher Katsu Takahashi expressed optimism, stating, “We want to help those suffering from tooth loss or absence. While no treatment to date has provided a permanent cure, we believe people’s expectations for tooth growth are high.”
This revolutionary drug promises a new era in dental medicine, offering hope to millions worldwide who suffer from tooth loss.
Source: https://www.kyoto-u.ac.jp/en/research-news/2021-03-31



















Comments